Nessun risultato di contenuto corrisponde alla tua parola chiave.
Contenuto
Sei uscito con successo.
Non sei ancora registrato?
Surgical Asset Management
Sfide quotidiane nella centrale di sterilizzazione

Cliccando 'Confirm" dichiari di essere un operatore sanitario.
Se sei un paziente o un giornalista visita le pagine a te dedicate.
Conferma Sì, sono un operatore sanitario. Cancella No, non sono un operatore sanitario.
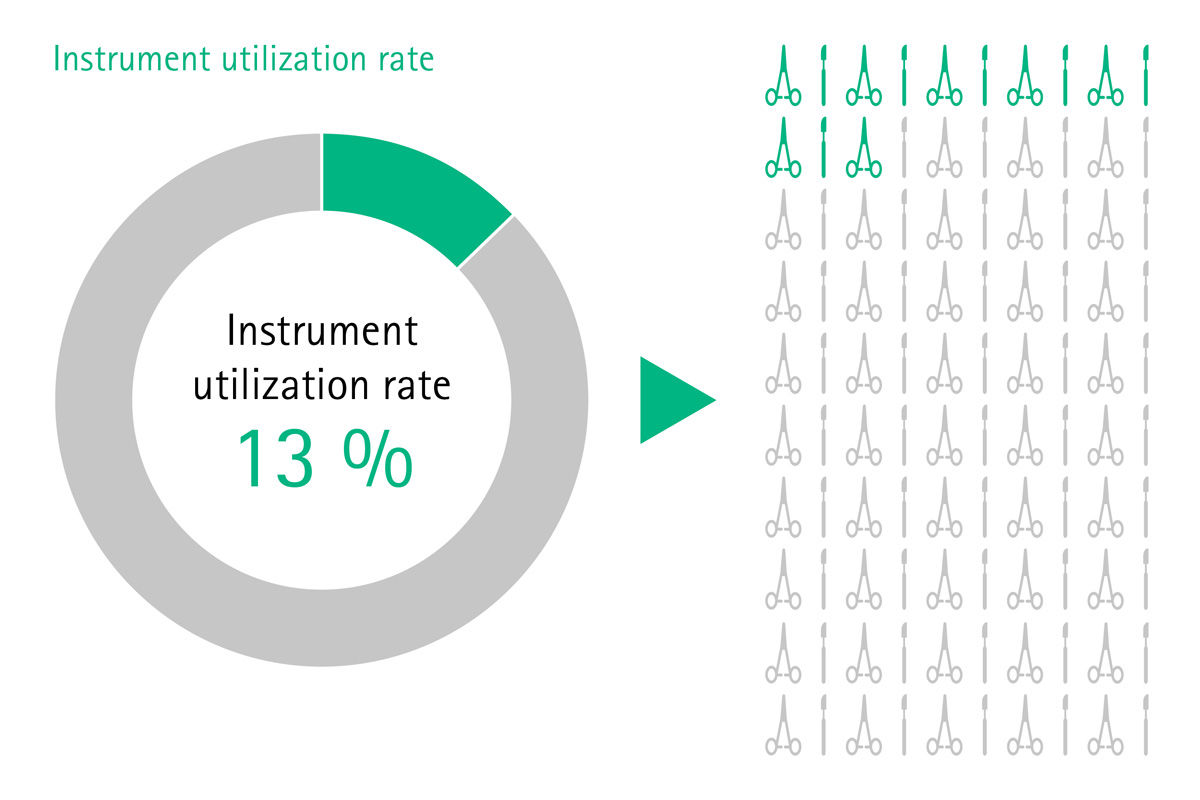

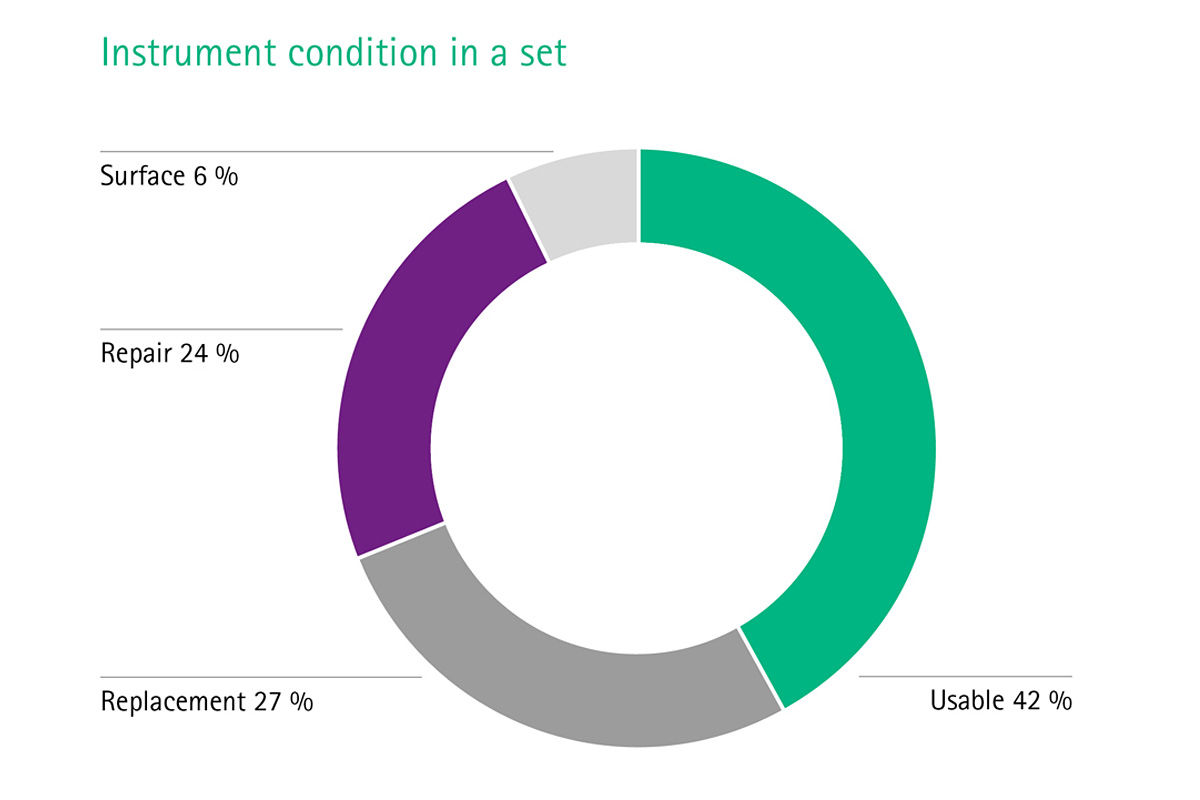
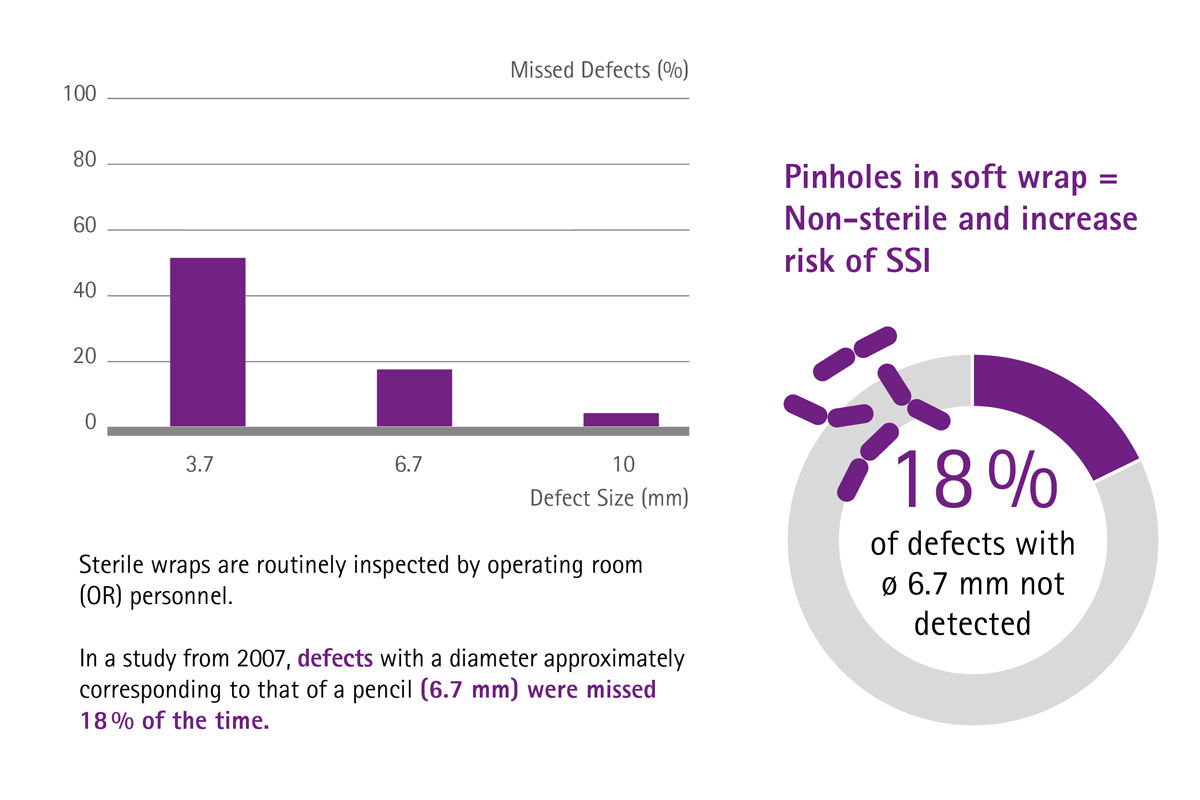





[1] Stockert EW, Langerman A. Assessing the magnitude and costs of intraoperative inefficiencies attributable to surgical instrument trays. J Am Coll Surg 2014;219(4):646-55.
[2] Paula JRdA, Silva RdCRd, Vedovato CA, et al. Instrumentais nas caixas cirúrgicas: avaliação de custo. Rev. Sobecc 2015;20(2):73-80.
[3] Guido Wismer, T.Z. (2013) Handbuch Sterilisation Von der Reinigung bis zur Bereitstellung von Medizinprodukten. Wiesbaden: mhp-Verlag GmbH
[4] Jelks, M.L. An overview of lean transformation in sterile processing. HEALTHCARE Purchasing News 2017:28-31
[5] Jeffreys B (2008). NHS ‘chaos’ over surgical tools. BBC, 24 April 2008.
[6] Aesculap. Data on file.
[7] Lunardini D, Arington R, Canacari EG, et al. Lean principles to optimize instrument utilization for spine surgery in an academic medical center: an opportunity to standardize, cut costs, and build a culture of improvement. Spine 2014;39(20):1714-17.
[8] Waked WR, Simpson AK, Miller CP, et al. Sterilization wrap inspections do not adequately evaluate instrument sterility. Clin Orthop Relat Res 2007;462:207-11.
[9] Aesculap. Data on file.
[10] Aesculap Sterile Technologies, Real time Event Related 360-day Shelf Life Study: STERILCONTAINER system with PrimeLine Lid, DOC570 Rev. B 3M 9/06.
[11] Aesculap, Sterilization Validation Study, One year Shelf-Life of the STERILCONATINERTM system with BASIC Lid, DOC 173 REV 5M 1/02.
[12] Junghanß U., Winterfeld S., Gabele L., Kulow U., Hygienisch-mikrobiologische und technische Überprüfung von Sterilisier-Containersystemen, Zentralsterilisation 1999, 7 (3):154-162.